TMCnet News
Scilex Holding Company Announces Publication in Anesthesiology Journal of Results from an Investigator-Initiated Research Study Using ZTlido® for the Treatment of Chronic Neck Pain¹
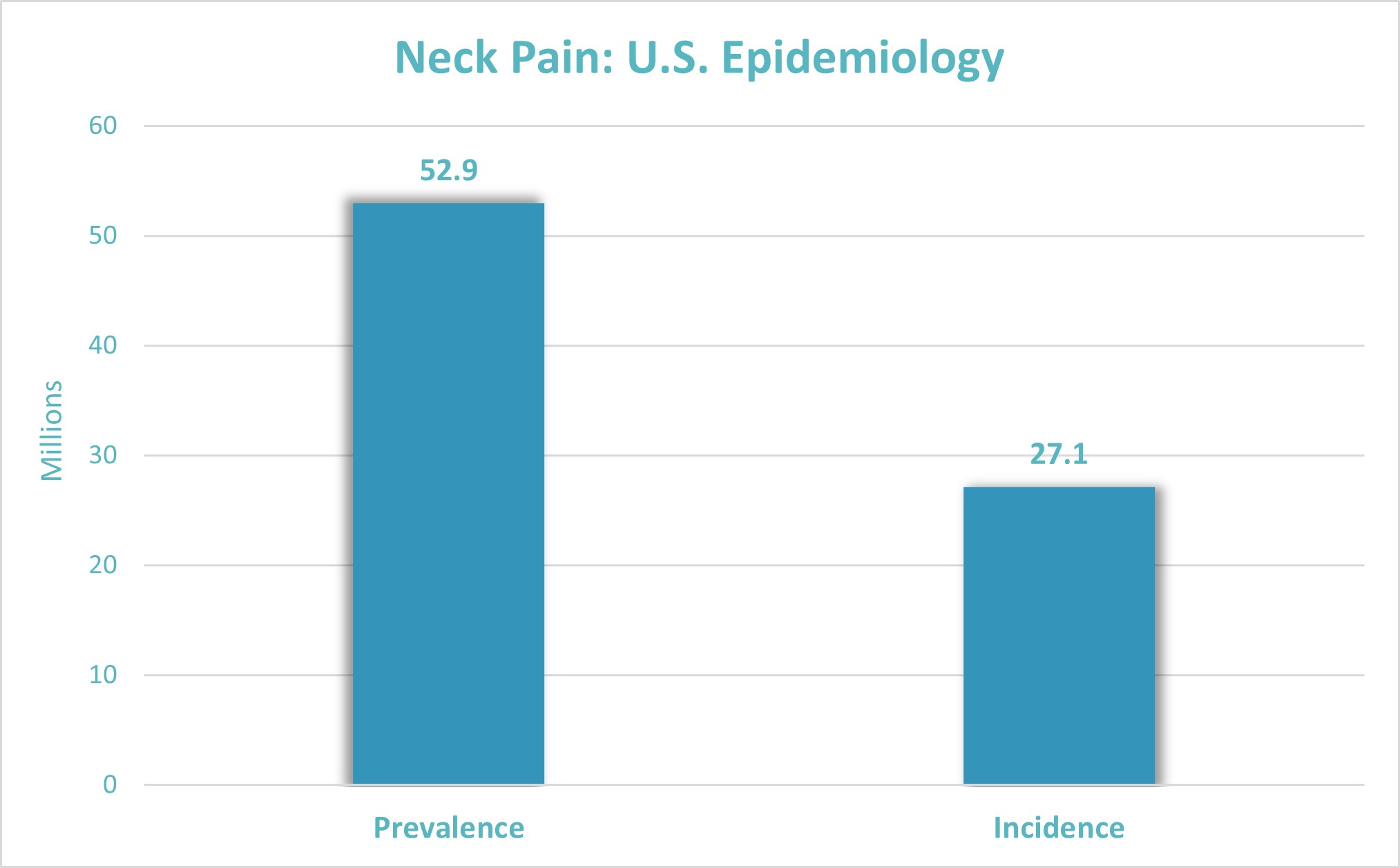
PALO ALTO, Calif., Feb. 20, 2024 (GLOBE NEWSWIRE) -- Scilex Holding Company (Nasdaq: SCLX, “Scilex” or “Company”), an innovative revenue-generating company focused on acquiring, developing and commercializing non-opioid pain management products for the treatment of acute and chronic pain, today announced publication in Anesthesiology Journal of results from an investigator-initiated research study using ZTlido® for the treatment of chronic neck pain. The lifetime prevalence of neck pain in the adult population is estimated to range from 14.2% to 71% with a mean of 48.5%.2 The global market for topical medications is projected to increase from $4.8 billion per year in 2021 to $7.3 billion per year in 2031.3 There are currently no approved medical treatments for neck pain in the U.S. The study was a randomized, double-blind, placebo-controlled crossover trial performed at four U.S. military, Veterans Administration, academic, and private practice sites, in which 76 patients were randomized to receive either a placebo patch followed by ZTlido® for 4-week intervals or vice versa. The primary outcome measure was mean reduction in average neck pain coupled with the Patient Global Impression of Change. The study demonstrated a clinical benefit trend for ZTlido® in treatment of neck pain, where 27.7% of patients experienced a positive outcome on ZTlido®, versus 14.9% on placebo. The effect was more pronounced during the first study period, reaching statistical significance. Analysis of both treatment periods did not show significant results due to the carry-over effect of the crossover study design and less than anticipated sample size due to early termination of the study (COVID pause and drug expiration). A positive trend was also observed for corresponding neck disability index. Overall, separation between ZTlido® and placebo effects and variability observed in this patient population provided necessary estimates for planning a potential Phase 3 development trial of SP-103 (triple-strength formulation of ZTlido®), which contains 3-times higher concentration of lidocaine. Additionally, this study provided essential insight into selection of patient population responsive to topical lidocaine treatment. Application of ZTlido® was well-tolerated and safety profile consistent with other ZTlido® trials previously conducted by Scilex. “Given the strong signal for clinically meaningful efficacy, our study results point to a potential necessity of further investigation of topical lidocaine treatment, especially in a formulation that contain higher dose and greater skin penetration than currently available alternatives, as it may provide greater clinical benefit to patients with chronic neck pain,” said Principal Investigator, Dr. Steven P. Cohen, Chief of Pain Medicine and Professor of Anesthesiology & Critical Care Medicine, Neurology, Physical Medicine & Rehabilitation, and Psychiatry & Behavioral Sciences at the Johns Hopkins School of Medicine, and Professor of Anesthesiology and Physical Medicine & Rehabilitation at Walter Reed National Military Medical Center, Uniformed Services University of the Health Sciences. “This proof-of-principle study data using ZTlido® in addition to our recently published skin penetration data demonstrating passive diffusion of lidocaine from SP-103 into a muscle layer, provide support for our strategy to pursue chronic neck pain indication for our next generation topical lidocaine system, aiming to become the first therapy approved for such common medical condition with significant unmet need for safe and effective non-addictive treatment,” said Dmitri Lissin, M.D., Chief Medical Officer of Scilex. Neck pain, or cervicalgia, is one of the most common pain presentations in U.S. and the 4th leading cause of disability. In the U.S., 52.9M adults suffer from Neck Pain.4 Prevalence of Neck Pain is estimated at greater than 20% of the adult population in the U.S. 4 In 2022, it was reported that neck pain was responsible for job absences among 25.5 million Americans, who missed an average of 11.4?days of work per year4. According to a 2020 JAMA publication (Journal of the American Medical Association), the U.S. low back and neck pain market is estimated t $134.5B.4
About Scilex Holding Company Scilex Holding Company is an innovative revenue-generating company focused on acquiring, developing and commercializing non-opioid pain management products for the treatment of acute and chronic pain. Scilex is uncompromising in its focus to become the global pain management leader committed to social, environmental, economic, and ethical principles to responsibly develop pharmaceutical products to maximize quality of life. Results from the Phase III Pivotal Trial C.L.E.A.R. Program for SEMDEXA™, its novel, non-opioid product for the treatment of lumbosacral radicular pain (sciatica), were announced in March 2022. Scilex participated in the type C meeting for purposes of pre-NDA discussion with the FDA and reached agreement on a path forward to file an NDA for SP-102 (SEMDEXA™) in Lumbosacral Radicular Pain (Sciatica) with the FDA. Scilex targets indications with high unmet needs and large market opportunities with non-opioid therapies for the treatment of patients with moderate to severe pain. Scilex launched its first commercial product ZTlido® in October 2018, in-licensed a commercial product Gloperba® in June 2022, and launched its third FDA-approved product Elyxyb® in April 2023. It is also developing its late-stage pipeline, which includes a pivotal Phase 3 candidate, and one Phase 2 and one Phase 1 candidate. Its commercial product, ZTlido® (lidocaine topical system) 1.8%, or ZTlido®, is a prescription lidocaine topical product approved by the U.S. Food and Drug Administration for the relief of pain associated with post-herpetic neuralgia, which is a form of post-shingles nerve pain. Scilex in-licensed the exclusive right to commercialize Gloperba® (colchicine USP) oral solution, an FDA-approved prophylactic treatment for painful gout flares in adults, in the U.S. Scilex in-licensed the exclusive rights to commercialize Elyxyb® (celecoxib oral solution) in the U.S. and Canada, the only FDA-approved ready-to-use oral solution for the acute treatment of migraine, with or without aura, in adults. Scilex launched Elyxyb® in April 2023, and is planning to commercialize Gloperba® by 2024, and is well-positioned to market and distribute those products. Scilex’s three product candidates are SP-102 (injectable dexamethasone sodium phosphate viscous gel product containing 10 mg dexamethasone), or SEMDEXA™, a Phase 3, novel, viscous gel formulation of a widely used corticosteroid for epidural injections to treat lumbosacral radicular pain, or sciatica, with FDA Fast Track status; SP-103 (lidocaine topical system) 5.4%, a Phase 2 study, triple-strength formulation of ZTlido®, for the treatment of chronic neck pain, with FDA Fast Track status in low back pain. We received our SP-103 Phase 2 top-line results in August 2023 and the trial achieved its objectives characterizing safety, tolerability and preliminary efficacy of SP-103 in acute low back pain associated with muscle spasms. SP-103 was safe and well-tolerated. Increase of lidocaine load in topical system by three times, compared with approved ZTlido, 5.4% vs. 1.8%, did not result in signs of systemic toxicity or increased application site reactions with daily applications over one month treatment. We will continue to analyze the SP-103 Phase 2 trial data along with a recently completed investigator study of ZTlido in patients with chronic neck pain which also has showed promising top-line efficacy and safety results. Scilex is planning to initiate Phase 2/3 trial in chronic neck pain in 2024; and SP-104, 4.5 mg Delayed Burst Release Low Dose Naltrexone Hydrochloride (DBR-LDN) Capsule, for the treatment of chronic pain, fibromyalgia that has completed multiple Phase 1 trial programs and is expected to initiate Phase 2 trials in 2024. Scilex Holding Company is headquartered in Palo Alto, California. Forward-Looking Statements This press release and any statements made for and during any presentation or meeting concerning the matters discussed in this press release contain forward-looking statements related to Scilex and its subsidiaries under the safe harbor provisions of Section 21E of the Private Securities Litigation Reform Act of 1995 and are subject to risks and uncertainties that could cause actual results to differ materially from those projected. Forward-looking statements include statements regarding the estimates for the prevalence of neck pain and the market size for low-back and neck pain market in the U.S., the results of the investigator-initiated research study and the potential for such results to support a potential Phase 3 development trial of SP-103, the potential market size and the size of the patient population for acute pain in the U.S., Scilex’s plans to initiate a Phase 2/3 trial in chronic neck pain in 2024 and plans to initiate Phase 2 trials in 2024 for SP-104, Scilex’s belief that it is well positioned to continue its growth over the next several years, Scilex’s long-term objectives and commercialization plans, Scilex’s potential to attract new capital, future opportunities for Scilex, Scilex’s future business strategies, the expected cash resources of Scilex and the expected uses thereof; Scilex’s current and prospective product candidates, planned clinical trials and preclinical activities and potential product approvals, as well as the potential for market acceptance of any approved products and the related market opportunity; statements regarding ZTlido®, Gloperba®, ELYXYB®, SP-102 (SEMDEXA™), SP-103 or SP-104, if approved by the FDA; Scilex’s development and commercialization plans; and Scilex’s products, technologies and prospects. Risks and uncertainties that could cause Scilex’s actual results to differ materially and adversely from those expressed in our forward-looking statements, include, but are not limited to: risks associated with the unpredictability of trading markets and whether a market will be established for Scilex’s common stock; general economic, political and business conditions; risks related to COVID-19 (and other similar disruptions); the risk that the potential product candidates that Scilex develops may not progress through clinical development or receive required regulatory approvals within expected timelines or at all; risks relating to uncertainty regarding the regulatory pathway for Scilex’s product candidates; the risk that Scilex will be unable to successfully market or gain market acceptance of its product candidates; the risk that Scilex’s product candidates may not be beneficial to patients or successfully commercialized; the risk that Scilex has overestimated the size of the target patient population, their willingness to try new therapies and the willingness of physicians to prescribe these therapies; risks that the outcome of the trials for SP-102, SP-103 or SP-104 may not be successful; risks that the prior results of the clinical and investigator-initiated trials of SP-102 (SEMDEXA™), SP-103 or SP-104 may not be replicated; regulatory and intellectual property risks; and other risks and uncertainties indicated from time to time and other risks set forth in Scilex’s filings with the Securities and Exchange Commission. Investors are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date of this release, and Scilex undertakes no obligation to update any forward-looking statement in this press release except as may be required by law. Contacts: Investors and Media Email: [email protected] Website: www.scilexholding.com Reference
Genebra et al. Prevalence and factors associated with neck pain: a population-based study SEMDEXA™ (SP-102) is a trademark owned by Semnur Pharmaceuticals, Inc., a wholly-owned subsidiary of Scilex Holding Company. A proprietary name review by the FDA is planned. ZTlido® is a registered trademark owned by Scilex Pharmaceuticals Inc., a wholly-owned subsidiary of Scilex Holding Company. Gloperba® is the subject of an exclusive, transferable license to use the registered trademark by Scilex Holding Company. ELYXYB® is the subject of an exclusive, transferable license to use the registered trademark by Scilex Holding Company. All other trademarks are the property of their respective owners. © 2024 Scilex Holding Company All Rights Reserved. 
|