TMCnet News
Top 3 Drivers of the Global Premature Ejaculation Treatment Market | TechnavioTechnavio market research analysts forecast the global premature ejaculation treatment market to grow at a CAGR of close to 9% during the forecast period, according to their latest report. This press release features multimedia. View the full release here: http://www.businesswire.com/news/home/20171203005057/en/ 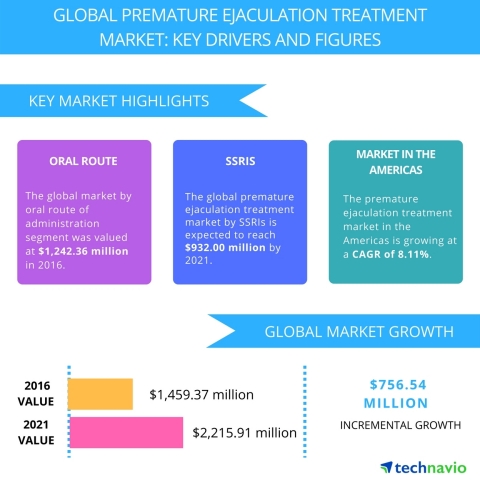
Technavio has published a new market research report on the global premature ejaculation treatment market from 2017-2021. (Graphic: Business Wire) The report further segments the global premature ejaculation treatment market by route of administration (oral route of administration and topical route of administration), by drug class (selective serotonin reuptake inhibitors (SSRIs), phosphodiesterase type 5 (PDE5) inhibitors, amide anesthetics, and others), and by geography (the Americas, EMEA, and APAC). This report is available at a USD 1,000 discount for a limited time only: View market snapshot before purchasing Buy 1 Technavio report and get the second for 50% off. Buy 2 Technavio reports and get the third for free. Technavio analysts highlight the following three market drivers that are contributing to the growth of the global premature ejaculation treatment market:
Looking for more information on this market? Request a free sample report Technavio's sample reports are free of charge and contain multiple sections of the report including the market size and forecast, drivers, challenges, trends, and more. High use of off-label drugs The global premature ejaculation treatment market has witnessed the increased use of off-label drugs that belong to different drug classes such as SSRIs (fluvoxamine, paroxetine, sertraline, and fluoxetine) and PDE5 inhibitors (Cialis, VIAGRA, and Levitra). Pharmacological modification of the ejaculatory response in patients with the condition represents a novel approach for the treatment of premature ejaculation. This will lead to the gradual replacement of psychosexual counseling that was previously regarded as the cornerstone of premature ejaculation treatment. Daily treatment with SSRIs has revolutionized the premature ejaculation treatment approach. According to Sapna Jha, a lead analyst at Technavio for men's health, women's health, and genitourinary research, "Drugs belonging to the SSRI drug class possess high efficacy with minor side effects. For instance, paroxetine 20-40 mg, sertraline 50-100 mg, fluoxetine 20-40 mg, and clomipramine 10-50 mg can be administered daily for premature ejaculation treatment. The anti-ejaculatory effect is achieved within five to ten days of administration of SSRIs. High efficacy of these drugs increases their demand among end-users, which has contributed to the growth of the market." Presence of unmet needs At present, there are only a few approved therapies that are available in the market for premature ejaculation indication. Some of them include Priligy, Tempe, Promescent, and EjectDelay. The low number of recognized drugs for premature ejaculation indication implies that a significant opportunity exists for new entrants to capitalize on the market. This existing need in the market is attracting several large pharmaceutical companies across the globe to capitalize on the market by investing in R&D programs and develop new solutions. "The development and regulatory approval of drugs from various regulatory authorities for the treatment of premature ejaculation will substantially decrease the dependence on off-label treatments such as PDE5 inhibitors that include tadalafil, sildenafil, and vardenafil. This will satisfy the unmet need for premature ejaculation treatment across the globe," says Sapna. Growth in strategic initiatives There has been an increase in the number of strategic initiatives in the premature ejaculation treatment market across the globe. This has significantly influenced the market growth. Typically, strategic initiatives involve partnerships and M&A between companies globally. They help the concerned companies to take advantage of the customer base, technology, financial capability, and R&D capabilities of their partners to increase their market shares and establish their presence in the global market. For instance, Innovus Pharma entered into a license and distribution agreement with Ovation in September 2013. As per the agreement, the company granted Ovation with an exclusive license to market and sell its product EjectDelay, a topical drug for premature ejaculation, in Morocco to expand its presence in EMEA. Browse Related Reports:
About Technavio Technavio is a leading global technology research and advisory company. Their research and analysis focuses on emerging market trends and provides actionable insights to help businesses identify market opportunities and develop effective strategies to optimize their market positions. With over 500 specialized analysts, Technavio's report library consists of more than 10,000 reports and counting, covering 800 technologies, spanning 50 countries. Their client base consists of enterprises of all sizes, including more than 100 Fortune 500 companies. This growing client base relies on Technavio's comprehensive coverage, extensive research, and actionable market insights to identify opportunities in existing and potential markets and assess their competitive positions within changing market scenarios. If you are interested in more information, please contact our media team at [email protected].
View source version on businesswire.com: http://www.businesswire.com/news/home/20171203005057/en/ |

