TMCnet News
Global Pharmaceutical Traceability Market - Growth Opportunity Assessment | TechnavioThe global pharmaceutical traceability market is expected to grow at a CAGR of more than 19% during the forecast period, according to Technavio's latest report. This Smart News Release features multimedia. View the full release here: http://www.businesswire.com/news/home/20170928005802/en/ 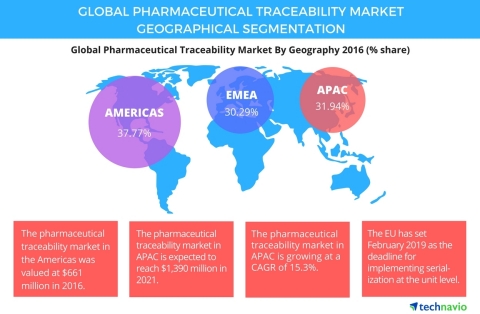
Technavio has published a new report on the global pharmaceutical traceability market from 2017-2021. (Graphic: Business Wire) In this report, Technavio covers the market outlook and growth prospects of the global pharmaceutical traceability market for 2017-2021. The market is segmented by type of technology (barcode, RFID, GPS, and others) and geography (the Americas, APAC, and EMEA). The demand for pharmaceutical traceability has grown over the years because of the rising concerns for global drug counterfeiting. The strict regulations from governments and vendors adopting new technologies have helped in improving the traceability of systems through track and trace and serialization. For example, companies like AlpVision have introduced a feature called Cryptoglyph, which uses a digital invisible marking on the labels. This marking is a digital image file that contains encrypted information that can be verified using a scanner and smartphone having the AlpVision application. Technavio's transportation and logistics research analysts categorize the global pharmaceutical traceability market into the following segments by regions:
Looking for more information on this market? Request a free sample report Technavio's sample reports are free of charge and contain multiple sections of the report including the market size and forecast, drivers, challenges, trends, and more. Americas: largest pharmaceutical traceability market The drug counterfeiting market in the Americas is small compared with other regions due to stringent policies by the government and various authorities. In 2013, FDA passed the Drug Supply Chain Security Act (DSCSA) to prevent counterfeiting in the pharmaceutical industry in the Americas. The act outlines the necessary steps to develop an electronic, interoperable system having the ability to trace, identify, and verify certain prescription drugs circulated in the market. "The government has taken initiatives to introduce regulations for implementing serialization at the unit level. The deadline for manufacturers, re-packagers, and re-labelers to abide by the new serialization law is November 2017. As per the law, each unit case should have serialized numerical identifiers at the serial level," says Shakti Jhakar, a lead analyst at Technavio for research on packaging. This report is available at a USD 1,000 discount for a limited time only: View market snapshot before purchasing Buy 1 Technavio report and get the second for 50% off. Buy 2 Technavio reports and get the third for free. Pharmaceutical traceability market in APAC China was the first country to introduce serialization in 2013. By February 2014, around 150 to 250 serialized batches were provided in the market. Serialization has been adopted at the unit, bundle, case, and pallet levels. The government generates the unique serial number for each drug. The pharmaceutical companies must request the Chinese government for obtaining the serial numbers, which are provided to the packaging lines. "In India, the Directorate General of Foreign Trade has made it mandatory for pharmaceutical products to have unique numbers and barcodes for tertiary, secondary, and primary packaging of all exports from the country by adopting the GS1 standards at different levels by November 2014," adds Shakti. Pharmaceutical traceability market in EMEA The European Union has set February 2019 as the deadline for implementing serialization at the unit level. The system is different from the US system because the serialization is applied at the front level and is verified only at the end point, excluding the intermediaries. According to the European Medicines Verification Systems (EMVS), manufacturers upload serialization information into a central website. In France, French CIP13 coding legislation initiated that all prescribed drugs include a specific data matrix code on the outer packaging from January 2011. The 2D matrix must contain the new CIP 13 code, batch number, and expiry date. The top vendors in the global pharmaceutical traceability market highlighted in the report are: Browse Related Reports:
About Technavio Technavio is a leading global technology research and advisory company. Their research and analysis focuses on emerging market trends and provides actionable insights to help businesses identify market opportunities and develop effective strategies to optimize their market positions. With over 500 specialized analysts, Technavio's report library consists of more than 10,000 reports and counting, covering 800 technologies, spanning across 50 countries. Their client base consists of enterprises of all sizes, including more than 100 Fortune 500 companies. This growing client base relies on Technavio's comprehensive coverage, extensive research, and actionable market insights to identify opportunities in existing and potential markets and assess their competitive positions within changing market scenarios. If you are interested in more information, please contact our media team at [email protected].
View source version on businesswire.com: http://www.businesswire.com/news/home/20170928005802/en/ |

