TMCnet News
The geko Device Makes its Debut at Major U.S. Orthopaedic ConferenceHIGH WYCOMBE, England, Jan. 28, 2020 /PRNewswire/ -- Sky Medical Technology Ltd, parent company of Firstkind Ltd, today announced that the geko™ device was presented as part of the didactic program as a case based presentation and at a corporate sponsored symposium at the Orthopaedic Summit 2019: Evolving Techniques (OSET 2019) annual meeting in Las Vegas.
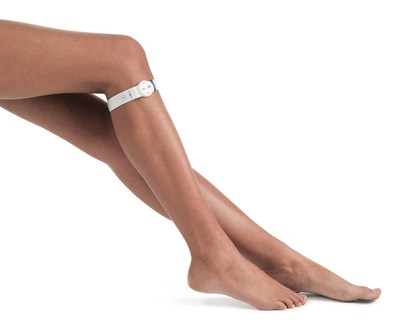
Jason Snibbe, MD, a fellow from the Kerlan-Jobe institute, USC team surgeon, and an orthopaedic surgeon practicing in Los Angeles, CA presented a real-world total knee replacement (TKR) patient case study to an audience of over 900 orthopaedic surgeons and healthcare professionals. The case focused on the patient's range of motion and mobility issues following total knee arthroplasty, where edema was determined as the culprit. Dr. Snibbe presented the clinical rationale and his use of the FDA cleared geko™ device to prevent the build-up of edema on the lower extremity orthopaedic procedures he performs. Dr. Snibbe later presented in a corporate sponsored symposium further information titled, Post-Operative Edema in Orthopaedics-Clinical Implications and an Innovative Solution along with Dr. Brian Loyd from the University of Utah. Post-operative edema occurs in over 90% of total hip and knee replacement patients1,2 and is one of the main post-operative challenges where there is no dependable solution. Attendees heard from faculty thought-leaders on the economic burden edema creates, as well as detail on this new, disruptive technology that provides a long needed solution for the orthopaedic community - the geko™ device - a wearable, edema management therapy. The geko™ device is the size of a wristwatch and worn at the knee. Through an innovative mechanism of bioelectronic neuromuscular electrostimulation, the daily disposable device gently stimulates the common peroneal nerve activating the calf and foot muscle pumps, resulting in increased blood flow in the deep veins of the calf for the prevention and treatment of pre and post-operative edema and the prevention of venous thromboembolism (VTE) - in both surgical and medical patients at risk of VTE. Practicing in Los Angeles area, Dr Jason Snibbe, is a board certified orthopedic surgeon, with fellowship training in Sports Medicine and Joint Replacement Surgery. He performs surgery on the shoulder, elbow, hip, and knee. His practice has a special focus on hip preservation and reconstructive surgery. He specializes in hip preservation techniques using advanced arthroscopic hip surgery. He also performs minimally invasive hip and knee replacements which allow patients an accelerated recovery. Dr Brian J Loyd, University of Utah, department of physical therapy, began his clinical research career, completing a Doctor of Physical Therapy degree at Texas Tech University Health Sciences Center. After earning his DPT, Loyd began his PhD program in rehabilitation science at the University of Colorado Anschultz Medical Campus with a focus on improving function and recovery in mobility limited populations. During his PhD training, Loyd was a contributing or first author on 7 peer-reviewed publications and the first author on 9 peer-reviewed conference abstracts. He completed this work under the mentorship of Jennifer Stevens-Lapsley, PT, PhD. The company is headquartered in the UK and backed by leading international investors in both healthcare and technology. To learn more about geko™ and using this device in the U.S., please contact Mark Sacaris at [email protected] or at 760.815.6474. Please also visit the geko™ website at: www.gekodevices.com. References: 1. K.L. Kluga et al Improving Orthopedic-Related Postoperative Edema Management in a Rehabilitative Nursing Setting. Rehabilitation Nursing, 44(3), 151–160. doi: 10.1097/rnj.0000000000000104. 2. B. J. Loyd et al. Development of a reference chart to monitor postoperative swelling following total knee arthroplasty. Disability and Rehabilitation, DOI: 10.1080/09638288.2018.1534005. - END - About Sky Medical Technology Ltd and Firstkind Ltd Sky Medical Technology, the parent of Firstkind Ltd, is a UK-based medical devices company. Through its innovative mechanism of wearable bioelectronic neuromuscular electrostimulation, Sky has developed a ground-breaking technology platform, OnPulse™, embedded in its industry-leading brand, the geko™ device. The company develops a range of products tailored to the needs of different medical application areas, selling both direct and through strategic partnerships or distributors in each major clinical area. Clinical areas of interest include DVT prevention, the prevention and treatment of edema and wound healing. The goal in each pathway is to partner with healthcare professionals to improve clinical outcomes and patient care whilst saving health system resources. Media contact (UK):
SOURCE Sky Medical Technology 
|