|
EPAM Debuts New Cloud-Powered Digital Clinical Trials Platform
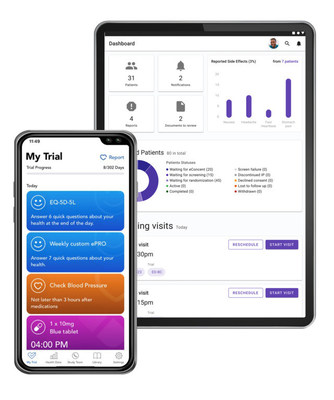
NEWTOWN, Pa., Nov. 17, 2021 /PRNewswire/ -- Clinical trials are a key step to bringing new pharmaceutical drugs to market, but they are also faced with an increasing number of challenges, from recruitment challenges to missed enrollment targets and patient dropouts. Accordingly, the pharmaceutical industry is quickly pivoting away from "site-centric" to "patient-centric" clinical trials. EPAM Systems, Inc. (NYSE: EPAM), a leading digital transformation services and product engineering company, announced today the launch of their new Digital Clinical Trials (DCT) platform. Powered by Microsoft cloud services, the solution is a digital, patient-centric platform that connects patients, Clinical Research Organizations (CRO), and healthcare provider teams to deliver the future of hybrid or decentralized clinical trials through a virtual, effortless, outcome-driven experience—making them more accessible, affordable, and faster.
CLOUD-BASED, MODULAR, INTEGRATED.
- Recruit Patients at Home with Digital Enrollment. Utilize remote screening at scale. Meet study enrollment targets faster and develop screening assessments to suit study needs and patient preferences.
- Clinical Trial Roadmap. Patients have an on-demand view into their personalized clinical trial experience at each step while they prepare for and manage their health.
- Omnichannel Communication. Bring secure, convenient, and reliable communication channels to patients wherever they are via telehealth, mobile messaging and/or eChat bots.
- Increase Compliance. Simple to use, connected devices that enable real-time patient outcome updates through integration.
- Connected Apps Dive Efficiency and Reduce Study Timelines. Accelerate study start-up timelines by facilitating easy document exchange between sponsor teams and trial site teams
"We're pleased to partner with Microsoft cloud services to bring the Digital Clinical Trials platform to market," said Sergey Yezhkov, SVP and Co-Head Global Business, EPAM Systems. "DCT gives pharmaceutical organizations access to real-time data via secure omnichannel communication channels, while simultaneously simplifying the patient recruitment and enrollment process, enabling seamless integration of patient outcomes, improving operational efficiencies, reducing study timelines, and increasing patient engagement."
"Clinical trials are an important element in bringing new drugs to market," said Daniel Carchedi, Sr. Director of Business Development & Strategy at Microsoft. "Together with EPAM, we are working to help our customers transform their businesses, and we're delivering impactful, human-centered solutions to the market every day."
Learn more about EPAM's new Cloud-Powered Digital Clinical Trials Platform at epam.com/DCT.
About EPAM Systems
Since 1993, EPAM Systems, Inc. (NYSE: EPAM) has leveraged its advanced software engineering heritage to become the foremost global digital transformation services provider – leading the industry in digital and physical product development and digital platform engineering services. Through its innovative strategy; integrated advisory, consulting and design capabilities; and unique 'Engineering DNA,' EPAM's globally deployed hybrid teams help make the future real for clients and communities around the world by powering better enterprise, education and health platforms that connect people, optimize experiences, and improve people's lives. Selected by Newsweek as a 2021 Most Loved Workplace, EPAM's global multi-disciplinary teams serve customers in more than 40 countries across five continents. As a recognized leader, EPAM is listed among the top 15 companies in Information Technology Services on the Fortune 1000 and ranked as the top IT services company on Fortune's 100 Fastest-Growing Companies list for the last three consecutive years. EPAM is also listed among Ad Age's top 25 World's Largest Agency Companies and in 2020, Consulting Magazine named EPAM Continuum a top 20 Fastest-Growing Firm. Learn more at http://www.epam.com and follow EPAM on Twitter and LinkedIn.
Forward-Looking Statements
This press release includes statements which may constitute forward-looking statements made pursuant to the safe harbor provisions of the Private Securities Litigation Reform Act of 1995, the accuracy of which are necessarily subject to risks, uncertainties, and assumptions as to future events that may not prove to be accurate. Factors that could cause actual results to differ materially from those expressed or implied include general economic conditions and the factors discussed in our most recent Annual Report on Form 10-K and other filings with the Securities and Exchange Commission. EPAM undertakes no obligation to update or revise any forward-looking statements, whether as a result of new information, future events, or otherwise, except as may be required under applicable securities law.
 View original content to download multimedia:https://www.prnewswire.com/news-releases/epam-debuts-new-cloud-powered-digital-clinical-trials-platform-301426393.html View original content to download multimedia:https://www.prnewswire.com/news-releases/epam-debuts-new-cloud-powered-digital-clinical-trials-platform-301426393.html
SOURCE EPAM Systems, Inc.

[ Back To TMCnet.com's Homepage ]
|