TMCnet News
KARL STORZ Announces New Non-Muscle Invasive Bladder Cancer Detection System: Photodynamic Diagnosis (PDD) Blue Light Flexible Video CystoscopyKARL STORZ Endoscopy-America, Inc., announces the commercial launch of the PDD Blue Light Flexible Video Cystoscopy System for enhanced detection of non-muscle invasive bladder cancer (NMIBC). This announcement follows the approval of a supplemental new drug application and a Premarket Approval supplement from the U.S. Food & Drug Administration (FDA) extending the indication for Blue Light Cystoscopy with Cysview® (BLCC) to include use of the new KARL STORZ PDD Blue Light Flexible Video Cystoscope. Also included in the approval is an expanded indication for the repetitive use of Cysview within the same patient and for the identification of Carcinoma in Situ (CIS); one of the most challenging types of bladder cancers to detect. These new indications greatly increase the treatment possibilities of this innovative therapy, which has rapidly become the standard of care for bladder cancer treatment at major medical institutions across the United States. This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20180518005055/en/ 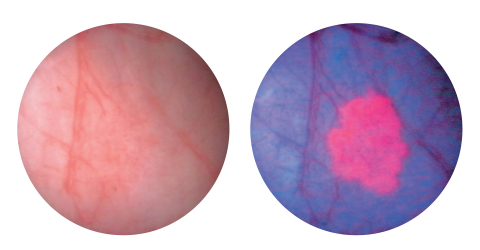
KARL STORZ PDD Blue Light Flexible Video Cystoscopy completes the 2-part system, which also includes Rigid Blue Light Cystoscopy. As shown above, Blue Light Cystoscopy with Cysview® enables cancerous tumors to fluoresce in a bright pink color, improving tumor visibility and enhancing florescence guided resection. (Graphic: Business Wire) Bladder Cancer affects over 708,444 patients in the US. Treatment is difficult because NMIBC tumors can look similar to normal healthy tissue, and can be missed or incompletely removed. Unfortunately, patients have a high probability of cancer recurrence, and in 2018 it is estimated that there will be 17,240 patient deaths due to bladder cancer1 KARL STORZ PDD Blue Light Flexible Video Cystoscopy completes the 2-part system, which also includes Rigid Blue Light Cystoscopy. The flexible and rigid PDD Blue Light Cystoscopy systems are designed to improve the visualization and resection of deadly NMIBC tumors. The new flexible system expands upon the rigid platform, enabling comfortable, anesthesia free examinations to confirm suspected lesions from a previous cystoscopy and for ongoing monitoring of NMIBC when used in conjunction with Cysview® an optical imaging agent manufactured and distributed in the U.S. by Photocure. Blue Light Cystoscopy with Cysview® enables cancerous tumors to fluresce in a bright pink color improving tumor visibility and enhancing florescence guided resection with the KARL STORZ UH 400 electrosurgical system. The FDA approval of KARL STORZ PDD Blue Light Flexible Cystoscopy with Cysview® (BLFCC) is based on results from a large Phase III study using the KARL STORZ blue-light-enabled rigid and flexible cystoscopes and blue light video system. During the Phase III clinical trial for Blue Light Flexible Cystoscopy, the following significant clinical findings were documented:2
"Use of the KARL STORZ PDD system for Blue Light Cystoscopy with Cysview® procedures continues to offer vital new capabilities to physicians," says John Martineau Director Marketing Urology, KARL STORZ Endoscopy-America, Inc. "It is also the only FDA-approved technology shown to improve detection of NMIBC tumors, leading to improved and more comprehensive florescence guided tumor resection. In the 2016 Guidelines for the management of NMIBC, the American Urological Association and Society for Urologic Oncology included Blue Light Cystoscopy as recommended for increasing the detection and reducing recurrence of NMIBC. We are certain that use of Blue Light Cystoscopy with Cysview® will be positioned to provide enhanced diagnostic and treatment capabilities to physicians and support improved outcomes for patients." "This expanded approval in both rigid and flexible blue light cystoscopy (BLC) means that Cysview® can now be used during transurethral resection of bladder cancer surgery for diagnosis and staging, as well as with follow-up surveillance of NMIBC. Patients with NMIBC, especially high grade, require careful and frequent follow-up due to the high rate of recurrence and progression. Blue light cystoscopy with Cysview®, will enable physicians to provide appropriate and more accurate treatment earlier, which in my experience results in improved outcomes for my patients. In my high-risk NMIBC clinical practice, I recognize the benefit of using BLC with Cysview® to more readily detect carcinoma in-situ, i.e. aggressive high-grade flat lesions. In this study, an additional 35% of CIS patients were found by using BLC with Cysview® alone and missed with white light," says Gary Steinberg, M.D., The Bruce and Beth White Family Professor, Vice Chairman and Director of Urologic Oncology, University of Chicago Medicine. About KARL STORZ KARL STORZ Endoscopy-America, Inc., is an affiliate of KARL STORZ SE & Co. KG, an international leader for more than 70 years in reusable endoscope technology, encompassing all endoscopic specialties. Based in Tuttlingen, Germany, KARL STORZ SE & Co. KG is a family-owned company that designs, engineers, manufactures, and markets all its products with an emphasis on visionary design, precision craftsmanship and clinical effectiveness. For more information, call 800-421-0837 or visit the company's Web site at www.karlstorz.com. About Photocure Photocure, The Bladder Cancer Company, delivers transformative solutions to improve the lives of bladder cancer patients. Our unique technology, which makes cancer cells glow bright pink, has led to better health outcomes for patients worldwide. Photocure is headquartered in Oslo, Norway, and listed on the Oslo Stock Exchange (OSE: PHO). The US headquarters for Photocure Inc., are in Princeton, New Jersey. For more information, please visit us at www.photocure.com, www.hexvix.com or www.cysview.com Cysview® is a registered trademark of Photocure, ASA 1 Cancer Facts and Figures 2017, American Cancer Society: Cancer Facts & Figure 2 Efficacy and Safety of Blue Light Flexible Cystoscopy with Hexaminolevulinate in the Surveillance of Bladder Cancer: A Phase III, Comparative, Multicenter Study; Sia Daneshmand, M.D., et.al: The Journal of Urology, ISSN: 1527-3792. Vol: 199. Issue: 5, Page: 1158-1165. View source version on businesswire.com: https://www.businesswire.com/news/home/20180518005055/en/ |

