TMCnet News
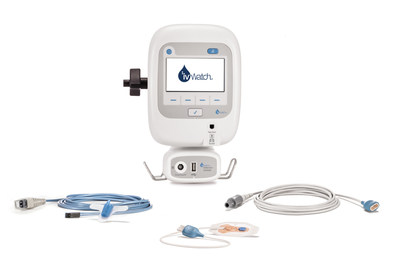
ivWatch Awarded Patient Safety Solutions Agreement with Premier, Inc.NEWPORT NEWS, Va., May 4, 2021 /PRNewswire/ -- ivWatch, LLC has been awarded a group purchasing agreement for Patient Safety Solutions with Premier, Inc. to provide more than 4,000 Premier member hospitals with greater access to the only continuous peripheral IV (PIV) site monitoring technology available in the market. The three-year agreement underscores the importance of detecting IV infiltration and extravasation events early to advance patient safety and create a new standard of care. The new agreement allows Premier members to take advantage of special pricing and terms pre-negotiated by Premier for the ivWatch Model 400 system. ivWatch's one-of-a-kind technology gives hospitals a continuous IV site monitoring solution with two sensor offerings – a reusable fiber optic sensor and a miniaturized disposable LED sensor. Both sensors measure changes in the optical properties of the tissue near an IV insertion site using visible and near-infrared light paired with a proprietary signal processing algorithm to help catch infiltrations early. ivWatch's IV Site Monitoring Technology Now Available to More Than 4,000 Premier Hospitals with Ne Three-Year Agreement
"We know that every infiltration is a drug delivery error and ivWatch exists to help eliminate patient harm associated with complications in IV therapy," said Gary Warren, President and CEO, ivWatch. "We are on a mission to help raise awareness about such an underreported, critical issue associated with IVs. We want to help advocate for patients and partner with hospitals and clinicians to help prevent IV infiltration and extravasation events from happening. We are excited to further our footprint in the United States to the network of Premier hospitals and provide easy access to ivWatch's innovative IV site monitoring solutions." Everyone is familiar with getting an IV, but not everyone knows about the estimated 46 million high-risk peripheral IVs given in the U.S. each year.1 ivWatch is here to partner with and notify clinicians in real-time of any tissue changes around an IV insertion site that indicate a potential infiltration and help hospitals deliver optimal outcomes in vascular therapy. Premier is a leading healthcare improvement company, uniting an alliance of more than 4,100 U.S. hospitals and approximately 200,000 other providers and organizations to transform healthcare. With integrated data and analytics, collaboratives, supply chain solutions, and consulting and other services, Premier enables better care and outcomes at a lower cost. To learn more about ivWatch monitoring tools, visit www.ivwatch.com or follow us on Facebook, Instagram, Twitter, and LinkedIn. 1 Fletcher Spaght, Inc. (2016) ivWatch Addressable Market: Analysis and Online Survey Results. About ivWatch, LLC
SOURCE ivWatch, LLC 
|